Unlock the benefits
of DILAPAN-S
Join other healthcare professionals who rely on DILAPAN-S for its proven, patient-centred approach.
Why to choose DILAPAN-S?
Reducing maternity
unit workload
Enjoy hospital staff time saving, minimal complications and efficient labour ward scheduling.
Efficacy
Discover reliable first-round cervical ripening success and vaginal delivery rate comparable to prostaglandins.
Safety
Benefit from minimal risk of hyperstimulation, reduced need for analgesia and no serious complications.
Maternal satisfaction
Take maternal satisfaction of your patients to a new level with gentle and comfortable cervical ripening.
Outpatient cervical ripening
Achieve a 70% vaginal delivery rate within 24 hours of admission, reduce hospital stays, and cut costs.
Versatility
Embrace ease with DILAPAN-S, the versatile choice suitable for a broad range of patient groups.
What users say
about DILAPAN-S?
Trusted by expectant mothers and doctors around the world.
Healthcare Professional, Lead Midwife, Cardiff UK
Healthcare Professional, Midwife, Nottingham, UK
Healthcare Professional, Consultant obstetrician and gynaecologist, Calderdale, UK
Healthcare Professional, Midwifery Sister, London UK
Healthcare Professional, Midwifery Sister, London UK
Healthcare Professional, Lead Midwife, Cardiff UK
Healthcare Professional, Consultant obstetrician and gynaecologist, Essex, UK
Healthcare Professional, Matron at Royal Surrey, UK
Healthcare Professional, Midwife, North Cumbria, UK
Mother
Mother
Mother
Mother
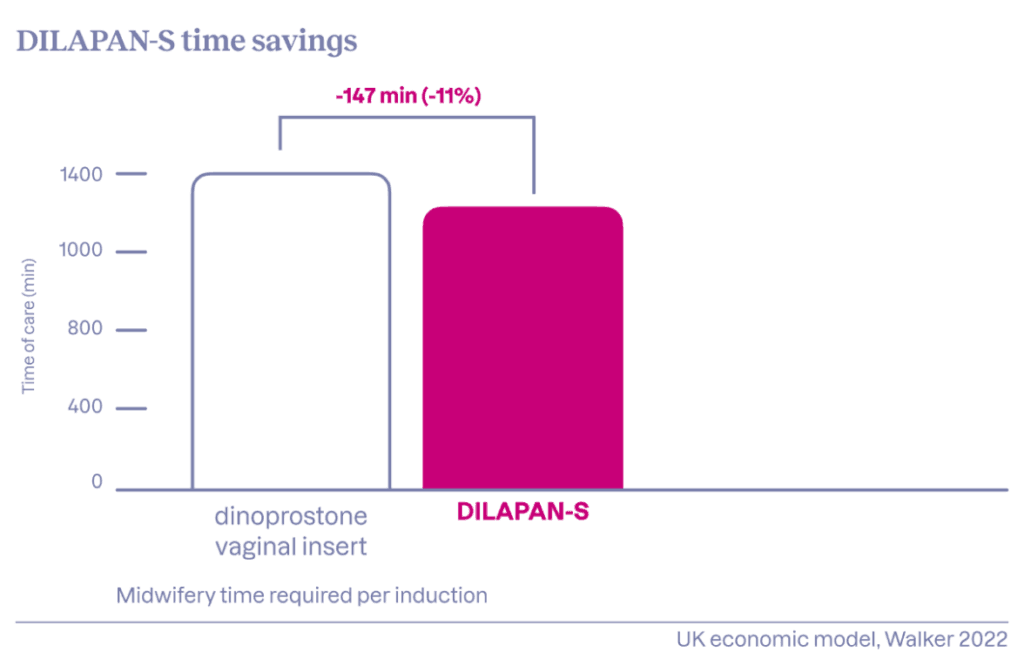
- Time savings of up to 2.4 hours per induction
- Minimal complications, eliminating the need for additional CTG monitoring
- Outpatient use with a low incidence of early admissions
- Predictability that allows for efficient labour ward scheduling
Women undergoing DILAPAN-S cervical ripening typically require minimal attention from hospital staff, allowing them to allocate their care to other patients. The gentle mode of action minimizes the risk of uterine hyperactivity and eliminates the need for repeated CTG monitoring or vaginal examinations. The unique hydrogel ensures consistency and predictability, achieving a cervical ripening success rate of approximately 80% over a 12–15 hour period.
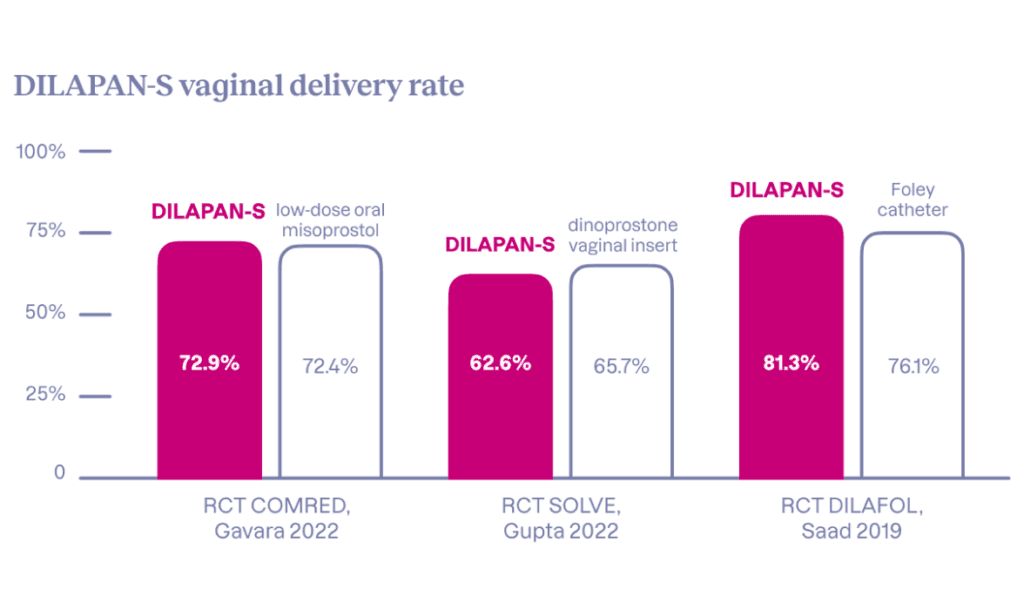
- Achievable first-round cervical ripening success at around 80%
- Vaginal delivery rates up to approximately 80%
- VD24* rate on par with low-dose oral misoprostol
Clinical evidence suggests that about 4 out of 5 women may achieve successful ripening with the first series of DILAPAN-S. In comparison to prostaglandins, DILAPAN-S provides a comparable vaginal delivery rate, including delivery within 24 hours*, while offering a better safety profile and enhanced maternal satisfaction.
The data indicate that the way DILAPAN-S is used and the entire IOL clinical protocol may impact the outcomes reached.
*VD24 rate—vaginal delivery rate within 24 hours
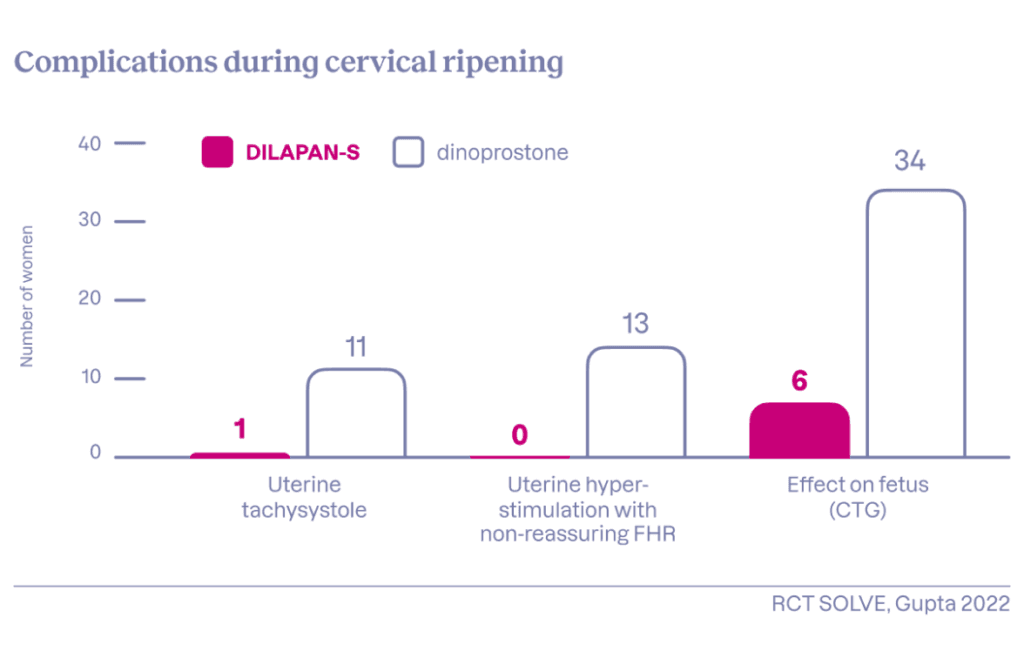
- Risk of uterine tachysystole minimised
- Significantly reduced need for analgesia
- No serious adverse maternal or neonatal outcomes
DILAPAN-S non-pharmacological cervical ripening can help manage the issue of frequent uterine tachysystole and the risk of hyperstimulation. A large randomised clinical trial (RCT SOLVE) found that DILAPAN-S may significantly reduce the need for analgesics (particularly strong opioids) compared with the dinoprostone vaginal insert. The NICE guideline encourages discussion with women about the safety benefits and risks of mechanical versus pharmacological methods.
- Superior to PGEs and balloon catheter
- DILAPAN-S offers the possibility to relax, move, or sleep
- Less pain, significantly reduced need for analgesia
Across clinical trials that compared DILAPAN-S with dinoprostone, misoprostol, or balloon catheters, women reported superior satisfaction with the cervical ripening procedure using DILAPAN-S. The outcomes confirm DILAPAN-S as one of the gentlest cervical ripening agents, maintaining efficacy and providing additional safety benefits.
